In a major breakthrough in energy storage, scientists in the United States have designed a new record-setting carbon “supercapacitor” material capable of storing four times more energy than the best commercial material currently available.
A supercapacitor made from this new material could store more energy, improve regenerative braking, power electronics and auxiliary power supplies.
” By combining a data-driven approach and our research experience, we created a carbon material with improved physicochemical and electrochemical properties that pushed the energy storage frontier for carbon supercapacitors to a new level. said chemist Tao Wang of ORNL and the University of Tennessee, Knoxville.
Wang led the study published in Nature Communications with chemist Sheng Dai of ORNL and UTK. ” This is the highest storage capacity recorded for porous carbon added Dai, who designed and performed the experiments with Wang. ” This is a real milestone. »
The benefit of machine learning
The ORNL-led study used machine learning, a type of artificial intelligence that learns from data, to optimize results to lead to the discovery of superlative material. Runtong Pan, Musen Zhou and Jianzhong Wu of the University of California, Riverside built artificial neural network model and trained him to set a clear goal: to develop “dream material» for energy supply.
The model predicted that the highest capacitance of a carbon electrode would be 570 farads per gram if the carbon was doped with oxygen and nitrogen. Wang and Dai designed an extremely porous doped carbon that would provide huge surface areas for interfacial electrochemical reactions.
Synthetic
Ultimately, the synthesized material had a capacitance of 611 farads per gram, four times that of a typical commercial material. PUSH pseudocapacitancewhich is charge storage based on continuous, fast and reversible redox reactions on the surface of electrode materials, contributed 25% to the total capacity.
The surface area of the material was among the highest recorded for carbonaceous materials, more than 4,000 square meters per gram. This success came quickly. The data-driven approach allowed the researchers to accomplish in three months what would previously have taken at least a year.
” We have reached the limit of the performance of carbon materials Wang said. ” Without the goal that machine learning has set for itself, we would continue to optimize materials through trial and error without knowing their limits. »
This research has the potential to accelerate the development and optimization of carbonaceous materials for applications supercapacitors. Although this groundbreaking study used the best data available at the time, the researchers now have even more frontier data to train a machine learning model for the next study.
” With more data, we can set a new goal and push the limits of carbon supercapacitors even further Wang said. ” The successful application of machine learning in material design is a testament to the power of data-driven approaches to advancing technology. »
For better understanding
1. What is a supercapacitor?
A supercapacitor is an energy storage device that can store more charge than traditional capacitors and can charge and discharge faster than batteries. They are often used in applications such as regenerative brakes, power electronics and auxiliary power supplies.
Machine learning is a type of artificial intelligence that learns from data to optimize results. In this study, it was used to guide the discovery of a new supercapacitor material by setting a clear goal: to develop “dream material» for energy supply.
3. What is capacity and why is it important?
Capacitance is the ability to collect and store electric charge. This is important because it determines how much energy the supercapacitor can store. The higher the capacity, the more energy the supercapacitor can store.
4. What is pseudocapacitance?
Pseudocapacitance is charge storage based on continuous, rapid, reversible redox reactions on the surface of electrode materials. In this study, the pseudocapacitance contributed 25% of the total capacitance of the new material.
5. What are the advantages of this new supercapacitor material?
This new material has a capacitance of 611 farads per gram, four times that of a typical commercial material. In addition, its surface area is among the highest recorded for carbonaceous materials, more than 4,000 square meters per gram. This means that it can store large amounts of energy and could therefore improve the performance of supercapacitors in various applications.
The main lesson
| Learning |
|---|
| A new supercapacitor material has been developed that can store four times more energy than the best current commercial material. |
| Machine learning was used to discover this material. |
| The material has a capacity of 611 farads per gram. |
| The pseudo capacity contributed 25% to the total capacity of the material. |
| The surface area of the material is among the highest recorded for carbonaceous materials, more than 4,000 square meters per gram. |
| The material was designed to have a combination of mesopores and micropores, allowing for a large surface area for energy storage and channels for electrolyte transport. |
| The research was completed in just three months using a data-driven approach that would normally take at least a year. |
| This research has the potential to accelerate the development and optimization of carbon materials for supercapacitor applications. |
| The researchers now have more data to train a machine learning model for further study. |
| The successful application of machine learning in material design is a testament to the power of data-driven approaches to advancing technology. |
Reference
Wang, T., Dai, S., et al. (2023). Record-breaking carbon supercapacitor material controlled by machine learning. The nature of communication. https://www.nature.com/articles/s41467-023-40282-1
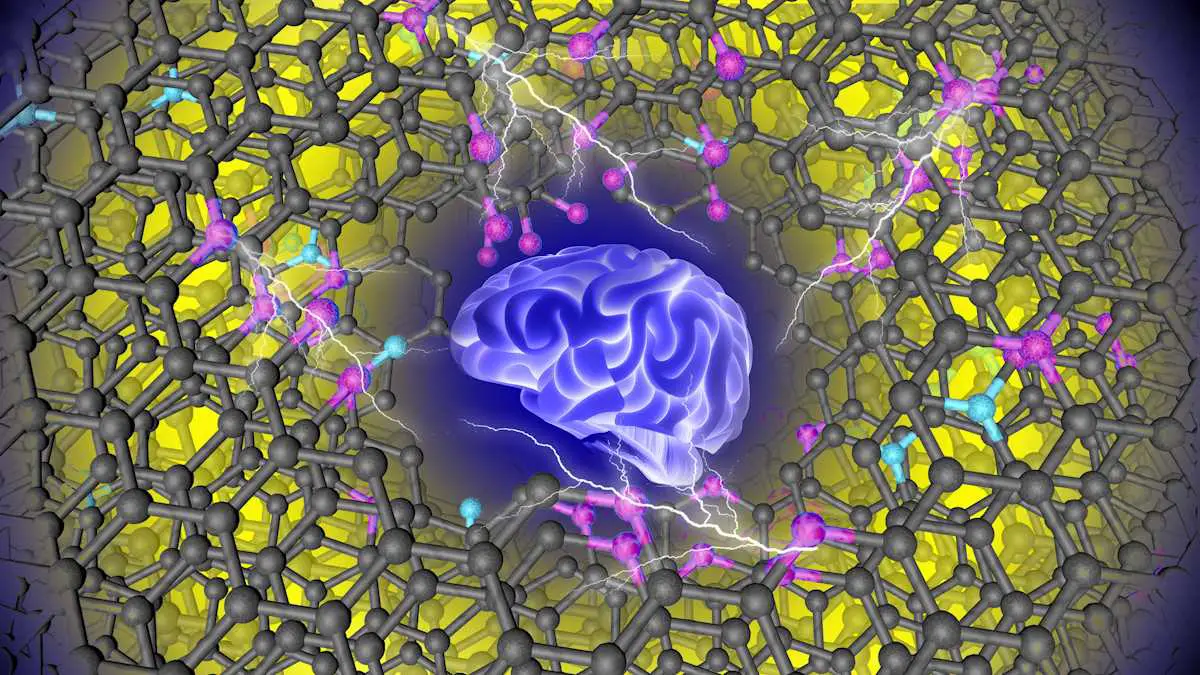
Main illustration caption: Concept art shows that machine learning has found the ideal material for capacitive energy storage. Its carbon structure, shown in black, includes functional groups with oxygen in pink and nitrogen in turquoise. Credit: Tao Wang/ORNL, US Dept. energy
(writing)